2. 内蒙古自治区新药筛选工程研究中心, 呼和浩特 010000;
3. 内蒙古医科大学新药安全评价研究中心, 呼和浩特 010000
2. Inner Mongolia Autonomous Region Engineering Research Center of New Pharmaceutical Screening, Hohhot 010000, China;
3. New Drug Safety Evaluation Research Center, Inner Mongolia Medical University, Hohhot 010000, China
多环芳烃类(Polycyclic aromatic hydrocarbons, PAHs)是常见的空气污染物,主要来源于烟草烟雾、煤炭燃烧、汽车尾气和室内烹调油烟,易在水中、土壤和作物中残留蓄积,是影响人体健康的大气颗粒物(Particulate matter, PM)主要的载体成分[1]。苯并[α]芘(Benzo[α]pyrene, BaP)是PAHs中具有代表性的物质,具有致癌、致畸、致突变和干扰内分泌等作用,BaP进入人体后,经细胞微粒体中Ⅰ相代谢酶P450的催化在7,8碳位上形成环氧化物,然后被微粒体环氧化物水解酶水解为二氢二醇,二氢二醇化合物在细胞色素P450催化下,进一步形成7,8-二氢二羟基-9,10-环氧化苯并[α]芘(Benzo[α]pyrene-trans-7, 8-diol-9, 10-epoxide, BPDE)。BPDE分子结构具有亲电性碳原子活性基团,可与核酸碱基和蛋白质氨基酸的亲核基团共价结合形成加合物,构成癌变的物质基础,BaP在代谢转化过程中通过不同途径对人体产生不良影响[2-3]。多氯代二苯并-对-二噁英(Polychlorinated dibenzo-para-dioxins, PCDDs)是PAHs不完全燃烧产物,广泛分布于环境中。它们是各种工业过程和废物焚烧的副产品。毒性最高的PCDD同源物是2,3,7,8-四氯二苯并-对-二噁英(Tetrachlorodibenzo-p-dioxin, TCDD)[4-5],可以引起皮肤、肝脏以及生殖系统和心血管系统的损害,而且具有潜在的致癌性[6-7]。大量研究表明,PAHs及其不完全燃烧产物在机体内依赖于芳香烃受体(Aryl hydrocarbon receptor, AhR)信号途径的激活和AhR反应基因的转录表达产生毒性[8-9]。由于BaP高度脂溶性的特点,极易透过细胞膜进入细胞质,在细胞质内作为配体与AhR结合,引起AhR蛋白构象改变,使得AhR与芳香烃受体核转运蛋白(Aryl hydrocarbon receptor nuclear translocator protein, ARNT)结合进入细胞核内并识别AhR反应基因上游部位的外源性反应元件(Xenobiotic response element, XRE),并与之结合启动下游基因的转录表达,产生相应的毒性反应。AhR的激活可能上调细胞色素P450(CytochromeP450, CYP450)代谢酶的表达,导致活性氧(Reactive oxygen species, ROS)的大量产生。在生理状态下,ROS在维持细胞和线粒体信号传导以及功能中发挥重要的作用,过量的ROS也可以引起组织氧化和细胞损伤,形成炎症和氧化应激的恶性循环,加剧疾病的发展进程[10]。近年来研究发现,核因子E2-相关因子2(Nuclear factor erythroid 2-related factor 2, Nrf2)在体内的抗氧化应激防御以及维持氧化还原稳态中发挥了重要的作用,其与基因启动子区域中的抗氧化反应元件(Antioxidant response element, ARE)结合调控一系列内源性抗氧化酶、Ⅱ相解毒酶及药物转运泵等相关基因的表达,发挥抗炎、抗氧化、抗细胞凋亡、抗细胞损伤的作用[11]。Nrf2的持续激活会造成癌细胞的生长,成为癌细胞逃避化疗药物攻击的一个途径,给癌细胞产生耐药性提供了条件[12]。
AhR作为一种配体激活的转录因子,在连接外源性化学刺激与适应性反应方面发挥重要作用,如解毒、维持机体平衡以及免疫反应等[13]。AhR参与调节物理、化学的外源性刺激引起的体内代谢,同时也在肿瘤形成、胚胎发育畸形及皮肤损伤中发挥作用[13-15]。Nrf2维持细胞内氧化还原平衡,在细胞周期调控和能量代谢等细胞基本生理过程中发挥重要作用。研究表明Nrf2直接调控细胞增殖[16]、调控细胞代谢水平[17]、调节线粒体功能[18]、影响细胞寿命[19]以及参与炎症反应[20]。当AhR、Nrf2沉默时,导致脂质堆积[21]、氧化损伤[22]及免疫失调[23],增加致癌易感性[24]。机体受到外源性有毒物质刺激时,AhR与Nrf2参与细胞防御、起到有效排毒以及维持机体内环境平衡的作用。然而AhR、Nrf2过表达时会造成机体的氧化应激、影响脂肪形成、糖原积累和相关耐药基因的表达,引起脂肪肝等疾病以及对化疗药物产生耐药性,对恶性肿瘤患者的预后不利。当前研究认为,机体受到外源性异物质刺激时,体内AhR可以有效帮助机体发挥解毒保护的功能,但机体持续接触外源性异物质,会导致AhR过表达,在机体氧化应激、肿瘤形成与发展方面产生不利影响。Nrf2是发挥机体抗氧化调节作用的关键因子,大量研究提示AhR与Nrf2之间可能存在复杂的作用机制,因此本文对AhR、Nrf2及其相互作用的分子机制进行系统综述。
1 AhR简介 1.1 AhR蛋白结构特征AhR是一种配体激活的转录因子,属于碱性螺旋-环-螺旋(Basic helix-loop-helix, bHLH)的Per-ARNT-Sim同源(Per-ARNT-Simhomolo-gydomain, PAS)家族的转录因子。AhR蛋白有3个功能结构域:bHLH结构域,PAS结构域和1个富含谷氨酸的结构域。bHLH结构域位于AhR蛋白的N-末端,包含用于核质穿梭的核定位序列(Nuclear localization sequence, NLS)和核输出信号(Nuclear export signal, NES),辅助AhR结合到靶基因的启动子区域和蛋白质二聚化。NLS上有三个蛋白激酶C(Protein kinase C, PKC)的磷酸化序列:S12、T22、S36,其中S12和S36的磷酸化,负调控AhR的入核行为,并影响AhR与DNA的结合能力;NES附近残基的磷酸化,可调节AhR在细胞中的定位。PAS结构域由两个不完整的重复序列PAS-A和PAS-B组成,可以增强AhR与ARNT异源二聚体的稳定性,并且PAS-B部分与配体结合结构域(Ligand binding domain, LBD)重叠。C-末端区域是一个富含谷氨酸的结构域, 发挥募集、转录激活的作用[25]。AhR基本结构(见图 1)。
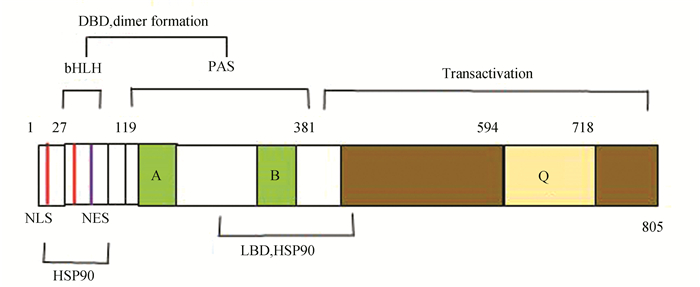
|
图 1 AhR结构示意图 Figure 1 AhR structure diagram 注:特征结构域是碱性螺旋-环-螺旋(bHLH),Per-ARNT-Sim(PAS)和反式激活域.DBD:DNA结合域;NLS:核定位信号;NES:核输出的信号;LBD:配体结合结构域以及HSP90相互作用结构域;A、B:弱同源重复区域;Q:谷氨酰胺丰富的转录激活结构域. |
AhR在胞浆内与多个信号蛋白相互作用,多种调控机制影响细胞信号传导,包括:ARNT、热休克蛋白(Heat Shock Proteins 90, HSP90)、乙型肝炎病毒X相关蛋白(Hepatitis B virus X protein-associated protein 2, XAP2)、p23、一些蛋白激酶及磷酸激酶和共激活因子等。AhR还与激素受体、低氧、炎症相关的转录因子和视网膜母细胞瘤肿瘤抑制蛋白(Retinoblastoma tumor suppressor protein family, Rb)介导的信号通路有相互作用[26-27]。在没有外源性配体的情况下,AhR与HSP90、XAP2、p23在细胞质形成复合物,保持未激活状态[28]。HSP90是一种维持AhR配体结合构象的伴侣蛋白,使AhR维持在一种非活化的、可结合配体的状态,起稳定AhR构象及抑制AhR与ARNT二聚化的作用。HSP90与AhR的bHLH区域和PAS-B区域相互作用,导致AhR核定位序列隐藏,不能入核。当前研究表明,配体(BaP)与AhR结合后,对机体主要产生两大方面影响:(1)经典的AhR-XRE信号通路被激活;(2)对其它信号通路中细胞因子的影响,体现为无XRE调控的非经典信号通路相互作用。经典的AhR-XRE信号通路,配体与AhR在细胞质中结合后,使得AhR构象变化,HSP90等从复合物解离,暴露N末端NLS,AhR与ARNT在细胞浆形成复合物转运至核内与XRE结合,调节下游基因CYP1A1(Cytochrome P450 Family-1Subfamily-A Polypeptide-1),CYP1A2(Cytochrome P450 Family-1Subfamily-A Polypeptide-2),CYP1B1(CytochromeP450 Family-1Subfamily-B Polypeptide-1)等基因的表达[29-30];也有研究认为当AhR激活后,AhR移位进入细胞核,AhR-配体-ARNT形成的复合物与XRE结合激活CYP代谢酶的表达[31-33]。由于AhR功能的复杂多样性,所以关于AhR-ARNT结合方式仍然存在分歧,有待相关实验验证。无XRE调控的非经典信号通路, 主要从以下几个方面来体现[34]:(1)通过影响细胞周期、凋亡、细胞接触性抑制相互作用等生理过程, 改变细胞的存活和增殖; (2)核因子-κB(nuclear factor-k-gene binding, NF-κB)与AhR之间的交互作用促进AhR信号传导, 诱导CYP1A1等基因表达; (3)通过AhR-雌激素受体(estrogen receptor, ER)信号通路直接或间接地干扰基因转录,影响正常的内分泌活动,导致雌激素依赖性疾病的发生。AhR作为一种配体激活的转录因子,在所有组织中都有表达,尤其在肝脏、脂肪组织和支气管上皮细胞中高表达。有研究表明,在恶性肿瘤中,芳香烃受体呈现高表达,并且参与了肿瘤细胞的多个生理过程,其通过与其他蛋白相互作用,调控一系列与肿瘤代谢相关的基因表达和信号通路,从而影响肿瘤的发生发展进程[35]。
2 Nrf2简介 2.1 Nrf2蛋白结构特征Nrf2属于Cap-N-Color(CNC)转录因子家族成员,调节细胞内抗氧化应激反应的关键转录因子,能够激活内源性抗氧化应答。Nrf2蛋白在机体多种组织内均有表达,且该蛋白分子包含Neh1~Neh7的7个结构域[36]:(1)Neh1区域具有与DNA结合的功能;(2)Neh3、Neh4和Neh5与共激活因子结合,为Nrf2反式激活结构域;(3)Neh2、Neh6和Neh7均能调节Nrf2的稳定性,Neh2含有2个细胞质蛋白Kelch样环氧氯丙烷相关蛋白-1(Kelch-like ECH-Associated protein 1, Keap1)的结合位点,从而负调控Nrf2转录活性。Nrf2基本结构(见图 2)。
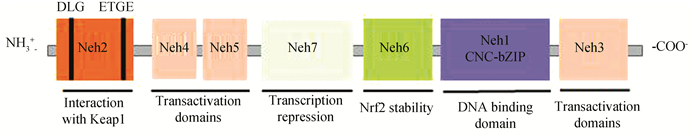
|
图 2 Nrf2结构示意图 Figure 2 Nrf2 structure diagram 注:Neh2结构域:抑制蛋白Keap1的结合位点,与Keap1同源二聚体在ETGE基序的高亲和性结合位点和一个与α螺旋区分离的DLG基序的低亲和性位点两个地方结合;Neh4和Neh5:转录激活域,与辅激活剂环磷酸腺苷(AMP)反应元件结合蛋白结合,并促进Nrf2转录,还与核辅助因子相互作用,增强Nrf2靶基因的表达;Neh7结构域与维甲酸X受体α相互作用,从而抑制Nrf2;Neh6结构域有一个与Keap1无关的负调控Nrf2的富含丝氨酸的区域;Neh1以及亮氨酸拉链基序使Nrf2与ARE序列结合;Neh3结构域的C末端与转录辅激活因子CHD6相互作用,CHD6是一种染色质重构后参与转活化的染色质ATP酶/螺旋酶DNA结合蛋白. |
Nrf2是调控细胞产生氧化应激反应的经典转录因子,诱导其下游产生一系列细胞保护蛋白, 维持细胞内环境稳态, 起到预防疾病发生的作用。在生理情况下,Nrf2被结合伴侣Keap1锚定在细胞质中,Keap1是依赖性泛素化连接酶E3蛋白(Cullin3, Cul3)复合物的底物,能够促使Nrf2发生泛素化且被蛋白酶体快速降解。当细胞受到ROS或亲电体的攻击时,细胞电位差发生变化,Keap1作为传感器感知,并产生信号失去与Nrf2相互作用活性,未被泛素化的Nrf2从Keap1中解离且快速转位进入细胞核[33, 37]。先与small Maf转录因子(Small Maf transcription factors, sMafs)蛋白形成异二聚体,再与ARE结合。活化的Nrf2与ARE结合时,还需与其他蛋白如JunD、cJun、转录激活因子4(Recombinant Activating Transcription Factor 4, ATF4)等形成异二聚体[38-39], 且需要协同转录因子参与才能激活过氧化氢酶(Catalase, CAT)、超氧化物歧化酶(Superoxide dismutase, SOD)、醌氧化还原酶1(NAD(P)H: quinone oxidoreductase-1, NQO1)、血红素氧化酶1(Heme oxygenase-1, HO-1)、UDP-葡萄糖醛酸基转移酶(UDP-glucuronosyltransferase, UGT)、谷胱甘肽-S-转移酶(Glutathione S-transferase, GST)等下游基因的转录[40]。目前已经鉴定出超过250个Nrf2靶基因,参与了多种细胞过程,例如:氧化还原调节、Ⅰ-Ⅲ相药物/异生物质代谢、蛋白质稳态、DNA修复、碳水化合物和脂质代谢、铁稳态、转录调节和线粒体功能等生物过程。尽管Nrf2通过其转录靶点直接调节许多细胞反应,但其也可通过与其他主要信号级联通路的串扰来调节细胞功能。这些通路对应激反应至关重要,包括Notch1、AhR、NF-κB、细胞肿瘤抗原p53、AMP依赖的蛋白激酶(Adenosine 5’-monophosphate (AMP)-activated protein kinase, AMPK)、磷脂酰肌醇-3激酶/蛋白激酶B(Phosphatidylinositol 3-hydroxy kinase/protein kinase B, PI3K-AKT)和雷帕霉素蛋白(Mammalian target of rapamycin, mTOR),突显了Nrf2在维持机体生理状态中起到关键作用。
Nrf2是调控细胞氧化应激的重要转录因子,同时也是维持细胞内氧化还原稳态的中枢调节者,Nrf2通过诱导调控一系列抗氧化蛋白的组成型和诱导型表达,可以减轻ROS和亲电体引起的细胞损伤,使细胞处于稳定状态,维持机体氧化还原动态平衡[41]。研究表明[42],Nrf2调控自噬基因的表达,如p62,SQSTM1基因。自噬通常是去除功能失调的细胞内成分所必需的过程,但在处理应激的细胞中,自噬的速率和程度都会增加,这与Nrf2的诱导相一致。此外,Nrf2与NF-κB信号通路具有拮抗作用,共同维持内环境的相对稳定[43]。在心肌细胞中,敲除Nrf2会加速缺血损伤后的心力衰竭[44]。针对皮肤、骨骼和角膜,Nrf2在伤口愈合中也有一定作用[45]。Nrf2信号通路的下调已被报道在心血管疾病如衰老、糖尿病、高血压和慢性炎症的风险增加。还有研究表明:与非肿瘤组织相比,肿瘤组织中Nrf2的表达水平明显增高。细胞内产生的过量ROS使得Nrf2过表达,导致结肠组织发生炎性反应,进而促进相关肿瘤的发生[46]。肿瘤细胞系或者肿瘤组织通过Keap1突变或基因杂合性丢失,上调Nrf2蛋白水平及其下游基因的转录,会促进肿瘤细胞的生长[47]。此外,还有研究表明Nrf2的过表达能够诱导肿瘤细胞产生化学耐药性从而导致肿瘤细胞的生长[48]。随着对Nrf2研究的逐渐深入,越来越多的证据表明,Nrf2在发挥积极作用的同时与肿瘤的形成以及耐药性的产生密不可分[49]。Nrf2能够促进肿瘤细胞产生耐药性,抑制Nrf2/ARE通路已成为肿瘤耐药性研究的热点问题[50]。
3 AhR与Nrf2信号通路的相互作用 3.1 AhR/Nrf2在毒性和解毒方面的相互作用AhR/Nrf2信号通路之间的紧密耦合可能大大降低CYP代谢酶生成的有毒中间体和ROS所带来的风险。有文献报道[51]通过AhR/Nrf2/NQO1通路能够有效抑制NLRP3炎性小体激活,改善小鼠结肠炎。Bose等人指出[52]当AhR、CYP1B1和Keap1的表达下调,随后保护性Nrf2、HO-1、NQO1和GSTA1表达增强,从而揭示通过AhR/CYP1B1/Nrf2/Keap1途径抑制肝癌发生的化疗潜力。大气污染中的PM导致组织氧化应激,引起心血管疾病、动脉粥样硬化等疾病以及加速皮肤老化,导致皮肤炎症和癌变。PM诱导的氧化应激可以上调Ⅰ期和Ⅱ期代谢酶的表达,这种上调是依赖AhR和Nrf2共同调节的,从而使机体得到较大程度的缓解[53];也可以通过调控AhR/CYP1A1/ROS通路激活Nrf2/HO-1[54-55]。Yoko等人研究发现[56]通过AhR-Nrf2通路可以有效抑制角质形成保持皮肤的动态平衡,抵抗氧化应激和减缓皮肤老化。还有研究发现,Nrf2通路被激活,能够保护心功能,减少梗死面积,减少氧化应激和炎症反应,AhR也参与了保护作用,形成一种长期的保护机制[57]。关于AhR与Nrf2之间复杂的作用机制以及对疾病基因的影响还需深入研究,通过干扰AhR-ARNT-XRE、Keap1-Nrf2-ARE信号通路,以期为抗氧化治疗提供新思路,为恶性肿瘤基因治疗研究提供了一个重要策略,为防治多种疾病提供重要的参考[58]。
3.2 Nrf2-ARE对AhR信号在脂肪代谢中的相互作用AhR可以通过多种途径调控Nrf2以及下游基因的表达,使细胞维持正常生理活性,Nrf2也可以调控AhR及其代谢酶的表达进而影响脂肪分化。脂肪形成在多种疾病(例如肥胖症、糖尿病、癌症和心血管疾病)和脂肪营养不良中起着关键作用。AhR在参与CYP代谢酶调节的同时还对发育、凋亡、生长和脂肪生成等生物过程造成一定影响[59],通过影响在脂肪合成中起重要作用的过氧化物酶体增殖激活的受体2(Peroxisome proliferator actived receptor2, PPAR2)对脂肪的分化起负向调节作用。Shimba等[60]通过实验证明TCDD处理AhR基因敲除小鼠的胚胎成纤维细胞(Mouse embryonic fibroblast, MEF),有效抑制了3T3-L1前脂肪细胞向脂肪细胞的转化,AhR影响脂肪分化独立于XRE。Alexander等[61]报道,AhR是甘油三酯合成的抑制剂,是脂肪细胞分化的早期调节剂,AhR基因敲除小鼠表现出短暂性脂肪肝[62]。Nrf2也会参与脂肪分化的调控过程并通过调节AhR以及下游靶基因CYP代谢酶的表达来影响脂肪分化。Shin等人研究表明[63]沉默Nrf2会加速脂肪细胞的分化,而Keap1的破坏会延迟该过程,表明Nrf2负反馈调节脂肪形成。并且AhR组成型表达受Nrf2基因型影响,Nrf2直接与AhR启动子230 bp区域的ARE结合,由于AhR负向调控脂肪细胞的分化,因此Nrf2可能通过与AhR相互作用来抑制脂肪的形成。Miao等人通过实验证明[64],正常情况下,Nrf2的激活使AhR的mRNA表达量增加,同时诱导下游基因CYP代谢酶的表达。但当Nrf2基因敲除后,AhR以及下游靶基因无明显变化。即AhR基因的转录直接受Nrf2的影响,通过Nrf2与AhR的相互作用来抑制脂肪的形成。由以上研究结果可知,Nrf2通过直接调控AhR以及下游靶基因的表达来影响脂肪分化。
3.3 AhR对Nrf2信号通路的调控 3.3.1 AhR直接调控Nrf2的表达在肝肿瘤细胞内AhR可直接调控Nrf2的表达,TCDD与AhR结合后,诱导Nrf2的表达[65]。Guo等研究表明[66]:下调AhR后可降低BaP诱导的Nrf2表达。Miao等[64]也证实AhR直接调控Nrf2基因的转录,使用siRNA沉默AhR结果显示TCDD对Nrf2 mRNA的诱导显著下调,Nrf2的表达不仅受AhR的直接调控,还通过位于其启动子近端区域的ARE样反应元件调节其自身表达,进而导致Nrf2持续不断的核积累和对其靶基因的持久诱导[67]。Mitoma[68]等人当沉默AhR后,Nrf2核易位明显减弱,对Nrf2-NQO1信号通路产生影响。当AhR配体激活AhR后,机体代谢异常激活Nrf2信号通路,使机体免受有毒物质所造成的损伤。
3.3.2 内环境的改变间接激活Nrf2的表达以往研究表明:AhR-ARNT-XRE信号通路和Keap1-Nrf2-ARE信号通路未发现明显的相互作用。当前研究显示外源性物质(BaP)可激活上述两个通路,并且两条通路之间可能存在相互作用[6]。BaP与AhR结合后,激活AhR-ARNT-XREs信号传导途径,诱导代谢酶基因的转录表达。代谢产生的活性产物使细胞发生氧化应激(Oxidative Stress, OS),导致中性粒细胞炎性浸润,蛋白酶分泌增加,产生大量的ROS,进而激活抗氧化通路,使得Nrf2与Keap1解离并转移到细胞核,Nrf2与相关基因的ARE结合以增强细胞抗氧化能力,减少活性自由基的损伤[69]。代谢过程中产生的ROS对于AhR调控Nrf2表达起到至关重要的作用,Christoph等人指出[70], 环境中的PAHs及其不完全燃烧的产物,在机体内先与AhR结合后,激活了AhR信号通路,进而激活CYP1A1的表达后产生的ROS间接激活Nrf2的表达,外源性有毒物质诱导Nrf2的表达、核迁移和Nrf2蛋白的表达在动力学上迟于AhR[67]。Nebert等研究表明TCDD诱导线粒体ROS的产生依赖于AhR[71]。因此,TCDD可能通过激活AhR过程中产生ROS进而激活抗氧化反应元件Nrf2/ARE通路,使得NQO1表达上调。产生一系列抗氧化反应,使机体免受氧化反应损伤,启动细胞的自我保护机制[72]。
3.3.3 AhR通过下游通路间接激活Nrf2丝裂原活化蛋白激酶(Mitogen-activated protein kinase, MAPK)、PKC和PI3K等经典信号通路作为AhR-ARNT-XRE下游通路并且参与Nrf2-Keap1的调节[73-75]。通过分析氨基酸序列发现Nrf2的反式激活结构域含有MAPK蛋白磷酸位点[76]。Levy等人发现JNK磷酸化的Nrf2能增加人支气管上皮细胞Nrf2信号通路靶基因的表达[77];Wei等人证明PKC信号通路能够使得Nrf2与Keap1解离,通过干扰PKC通路可以降低Nrf2下游基因的表达水平[75]。Kay等人[78]通过使用RNAi技术以及PKC、c-Jun氨基末端激酶(C-Jun N-terminal kinase, JNK)和p38丝裂原活化蛋白激酶(p38 mitogen-activated protein kinase, p38MAPK)基因的抑制剂,结果显示抑制PKC、JNK、p38等基因的表达减少了Nrf2的核转运、降低ARE的活性,抑制了Nrf2基因的表达。AhR-ARNT-XRE与Keap1-Nrf2-ARE信号通路及其相互作用(见图 3)。
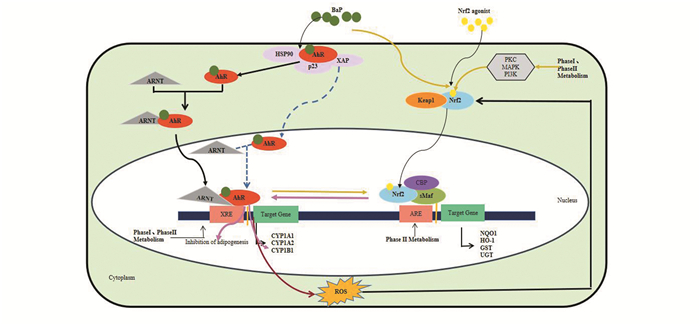
|
图 3 AhR、Nrf2以及AhR/Nrf2的分子机制示意图 Figure 3 Schematic diagram of molecular mechanism of AhR, Nrf2, and AhR/Nrf2 注:AhR信号通路通过AhR-ARNT-XRE调节下游靶基因的转录及表达.Nrf2抗氧化信号通路通过Nrf2-sMaf-ARE使机体免受氧化损伤。AhR/Nrf2相互作用:1.AhR直接激活Nrf2;2.通过代谢过程中产生的活性氧(ROS)从而激活Nrf2抗氧化通路;3.AhR通过下游PKC、MAPK、PI3K等信号通路可以影响Nrf2抗氧化通路;4.Nrf2可以通过调节AhR以及下游靶基因CYP代谢酶的表达来影响脂肪分化. |
综上所述,在没有多环芳烃化合物BaP配体存在下,大部分AhR与HSP90、XAP2、p23在细胞浆形成复合物,保持未激活状态;当机体受到BaP刺激后,AhR被激活,与ARNT在细胞浆形成复合物转运至核内与XRE结合激活CYP代谢酶的表达。在生理状态下,Nrf2被结合伴侣Keap1锚定在细胞浆中并促进Nrf2泛素化;当细胞受到ROS或亲电体的攻击时,Nrf2与Keap1解离,快速转位进入细胞核且与ARE结合,促进下游基因的转录与表达。AhR与Nrf2信号通路存在交互作用:(1)AhR直接激活Nrf2的表达;(2)通过代谢过程中产生的ROS激活Nrf2抗氧化通路;(3)AhR通过下游PKC、MAPK、PI3K等信号通路可以影响Nrf2抗氧化通路;(4)Nrf2可以通过调节AhR以及下游靶基因CYP代谢酶的表达来影响脂肪分化。
普遍表达于多种细胞的转录因子AhR、Nrf2介导的细胞解毒及抗氧化机制在对抗各种环境应激和内源性应激中发挥重要作用,是参与肿瘤预防及形成、肿瘤耐药、神经和血管保护的重要效应分子。虽然AhR在肿瘤发生发展中的作用已经取得了一定的研究成果,但其具体的致癌机制仍然存在较多疑问,有待于进一步的研究加以解释。Nrf2传统上是一种肿瘤抑制因子,是抵抗内源性和外源性氧化损伤的主要细胞防御机制,然而,Nrf2过度激活创造了一个有利于正常细胞和肿瘤细胞生长的环境,保护它们免受氧化应激、化疗药物和放射治疗的影响[79]。由于Nrf2在肿瘤的发生发展过程中具有两面性[80],进一步拓展蛋白质组学和代谢组学的分析也是研究Nrf2调控细胞增殖的有效策略,为癌症的治疗和预后以及靶向药物的使用提供了新的切入点。此外,Nrf2诱导肿瘤耐药的机制尚未完全阐明,有待进一步探索,以便更有针对性地抑制Nrf2在耐药肿瘤组织中的高表达[81]。因此,我们应深入研究AhR-Nrf2相互作用的分子机制,为进一步研究有毒物质在分子水平上解毒抗氧化的机制奠定基础。
| [1] |
NGUYEN P M, PARK M S, CHOW M, et al. Benzo[a]pyrene increases the Nrf2 content by downregulating the Keap1 message[J]. Toxicological Sciences, 2010, 116(2): 549-561. DOI:10.1093/toxsci/kfq150 (  0) 0) |
| [2] |
WANG H, PAN L, ZHANG X, et al. The molecular mechanism of AhR-ARNT-XREs signaling pathway in the detoxification response induced by polycyclic aromatic hydrocarbons (PAHs) in clam Ruditapes philippinarum[J]. Environmental Research Letters, 2020, 183: 109165. DOI:10.1016/j.envres.2020.109165 (  0) 0) |
| [3] |
WU Y, NIU Y, LENG J, et al. Benzo(a)pyrene regulated A549 cell migration, invasion and epithelial-mesenchymal transition by up-regulating long non-coding RNA linc00673[J]. Toxicology Letters, 2020, 320: 37-45. DOI:10.1016/j.toxlet.2019.11.024 (  0) 0) |
| [4] |
MARQUES M, NADAL M, DIAZ-FERRERO J, et al. Concentrations of PCDD/Fs in the neighborhood of a hazardous waste incinerator: human health risks[J]. Environmental Science and Pollution Research International, 2018, 25(26): 26470-26481. DOI:10.1007/s11356-018-2685-8 (  0) 0) |
| [5] |
GHOSH J, CHOWDHURY A R, SRINIVASAN S, et al. Cigarette smoke toxins-induced mitochondrial dysfunction and pancreatitis involves aryl hydrocarbon receptor mediated cyp1 gene expression: protective effects of resveratrol[J]. Toxicological Sciences, 2018, 166(2): 428-440. DOI:10.1093/toxsci/kfy206 (  0) 0) |
| [6] |
SVOBODOVA J, PROCHAZKOVA J, KABATKOVA M, et al. 2, 3, 7, 8-Tetrachlorodibenzo-p-dioxin (TCDD) disrupts control of cell proliferation and apoptosis in a human model of adult liver progenitors[J]. Toxicological Sciences, 2019, 172(2): 368-384. DOI:10.1093/toxsci/kfz202 (  0) 0) |
| [7] |
JOFFIN N, NOIREZ P, ANTIGNAC J P, et al. Release and toxicity of adipose tissue-stored TCDD: Direct evidence from a xenografted fat model[J]. Environment International, 2018, 121(2): 1113-1120. DOI:10.1016/j.envint.2018.10.027 (  0) 0) |
| [8] |
NATALIA K, RANCE N, ROBERT C, et al. Comparative analysis of TCDD-induced AhR-mediated gene expression in human, mouse and rat primary B cells[J]. Toxicology and Applied Pharmacology, 2017, 316: 95-106. DOI:10.1016/j.taap.2016.11.009 (  0) 0) |
| [9] |
CHEN Y, SHA R, LI X, et al. 2, 3, 7, 8-Tetrachlorodibenzo-p-dioxin promotes migration ability of primary cultured rat astrocytes via aryl hydrocarbon receptor[J]. Journal of Environmental Sciences, 2019, 76(02): 368-376. DOI:10.1016/j.jes.2018.05.030 (  0) 0) |
| [10] |
SHAH H K, SHARMA T, BANERJEE B D. Organochlorine pesticides induce inflammation, ROS production, and DNA damage in human epithelial ovary cells: An in vitro study[J]. Chemosphere, 2020, 246: 125691. DOI:10.1016/j.chemosphere.2019.125691 (  0) 0) |
| [11] |
PALLAB S, ANSUMAN C. Nrf2-ARE signaling in cellular protection: Mechanism of action and the regulatory mechanisms[J]. Journal of Cellular Physiologyvol, 2020, 235(4): 3119-3130. DOI:10.1002/jcp.29219 (  0) 0) |
| [12] |
CHIA A J L, GOLDRING C E, NEIL K R, et al. Differential effect of covalent protein modification and glutathione depletion on the transcriptional response of Nrf2 and NF-kappaB[J]. Biochemical Pharmacology, 2010, 80(3): 410-421. DOI:10.1016/j.bcp.2010.04.004 (  0) 0) |
| [13] |
ESSER C. Biology and function of the aryl hydrocarbon receptor: report of an international and interdisciplinary conference[J]. Archives of Toxicology, 2012, 86(8): 1323-1329. DOI:10.1007/s00204-012-0818-2 (  0) 0) |
| [14] |
IKUTA T, NAMIKI T, FUJⅡ K Y, et al. AhR protein trafficking and function in the skin[J]. Biochemical Pharmacology, 2009, 77(4): 588-96. DOI:10.1016/j.bcp.2008.10.003 (  0) 0) |
| [15] |
PANTELEYEV A A, BICKERS D R. Dioxin-induced chloracne-reconstructing the cellular and molecular mechanisms of a classic environmental disease[J]. Experimental Dermatology, 2006, 15: 705-730. DOI:10.1111/j.1600-0625.2006.00476.x (  0) 0) |
| [16] |
MURAKAMI S, MOTOHASHI H. Roles of Nrf2 in cell proliferation and differentiatio[J]. Free Radical Biology Medicine, 2015, 88: 168-178. DOI:10.1016/j.freeradbiomed.2015.06.030 (  0) 0) |
| [17] |
LEE S, HALLIS S P, JUNG K A, et al. Impairment of HIF-1α-mediated metabolic adaption by NRF2-silencing in breast cancer cells[J]. Redox Biology, 2019, 24: 101210. DOI:10.1016/j.redox.2019.101210 (  0) 0) |
| [18] |
DINKOVA K A T, ABRAMOV A Y. The emerging role of Nrf2 in mitochondrial function[J]. Free Radical Biology Medicine, 2015, 88: 179-188. DOI:10.1016/j.freeradbiomed.2015.04.036 (  0) 0) |
| [19] |
PAEK J, LO J Y, NARASIMHAN S D, et al. Mitochondrial SKN-1/Nrf mediates a conserved starvation response[J]. Cell Metabolism, 2012, 16(4): 526-537. DOI:10.1016/j.cmet.2012.09.007 (  0) 0) |
| [20] |
NAGASHIMA R, KOSAI H, MASUO M, et al. Nrf2 suppresses allergic lung inflammation by attenuating the type 2 innate lymphoid cell response[J]. Journal of Immunology, 2019, 202(5): 1331-1339. DOI:10.4049/jimmunol.1801180 (  0) 0) |
| [21] |
SHIN S, WAKABAYASHI J, YATES M S, et al. Role of Nrf2 in prevention of high-fat diet-induced obesity by synthetic triterpenoid CDDO-imidazolide[J]. European Journal of Pharmacology, 2009, 620(1/2/3): 138-144. DOI:10.1016/j.ejphar.2009.08.022 (  0) 0) |
| [22] |
CARVAJAL G J M, ROMAN A C, CEREZO G M I, et al. Loss of dioxin-receptor expression accelerates wound healing in vivo by a mechanism involving TGFbeta[J]. Journal of Cell Science, 2009, 122(11): 1823-1833. DOI:10.1242/jcs.047274 (  0) 0) |
| [23] |
THIMMULAPPA R K, LEE H, RANGASAMY T, et al. Nrf2 is a critical regulator of the innate immune response and survival during experimental sepsis[J]. The Journal of Clinical Investigation, 2006, 116(4): 984-995. DOI:10.1172/JCI25790 (  0) 0) |
| [24] |
RAMOS G M, KWAK M K, DOLAN P M, et al. Sensitivity to carcinogenesis is increased and chemoprotective efficacy of enzyme inducers is lost in nrf2 transcription factor-deficient mice[J]. Proceedings of the National Academy of Sciences of the United States of America, 2001, 98(6): 3410-3415. DOI:10.1073/pnas.051618798 (  0) 0) |
| [25] |
WANG J J, LEI KF, HAN F. Tumor microenvironment: recent advances in various cancer treatments[J]. European Review for Medical and Pharmacological Sciences, 2018, 22(12): 3855-3864. DOI:10.26355/eurrev_201806_15270 (  0) 0) |
| [26] |
BOCK K W, KOHLE C. Ah receptor: dioxin-mediated toxic responses as hints to deregulated physiologic functions[J]. Biochemical Pharmacology, 2006, 72(4): 393-404. DOI:10.1016/j.bcp.2006.01.017 (  0) 0) |
| [27] |
HESTERMANN E V, BROWN M. Agonist and chemopreventative ligands induce differential transcriptional cofactor recruitment by aryl hydrocarbon receptor[J]. Molecular and Cellular Biology, 2003, 23(21): 7920-7925. DOI:10.1128/mcb.23.21.7920-7925.2003 (  0) 0) |
| [28] |
SHAH S Z A, ZHAO D, HUSSAIN T, et al. p62-Keap1-NRF2-ARE Pathway: A contentious player for selective targeting of autophagy, oxidative stress and mitochondrial dysfunction in prion diseases[J]. Frontiers in Molecular Neurosciencevol, 2018, 11: 310. DOI:10.3389/fnmol.2018.00310 (  0) 0) |
| [29] |
LARIGOT L, JURICEK L, DAIROU J, et al. AhR signaling pathways and regulatory functions[J]. Biochimie Open, 2018(7): 1-9. DOI:10.1016/j.biopen.2018.05.001 (  0) 0) |
| [30] |
RAMOS G N A, OROZCO I M, ESTUDILLO E, et al. Aryl Hydrocarbon Receptor in Post-Mortem Hippocampus and in Serum from Young, Elder, and Alzheimer's Patients[J]. International Journal of Molecular Sciences, 2020, 21(6): 1983. DOI:10.3390/ijms21061983 (  0) 0) |
| [31] |
DENISON M S, SOSHILOV A A, HE G, et al. Exactly the same but different: promiscuity and diversity in the molecular mechanisms of action of the aryl hydrocarbon (dioxin) receptor[J]. Toxicological Sciences, 2011, 124(1): 1-22. DOI:10.1093/toxsci/kfr218 (  0) 0) |
| [32] |
GUERRINA N, TRABOULSI H, EIDELMAN D H, et al. The aryl hydrocarbon receptor and the maintenance of lung health[J]. International Journal of Molecular Sciences, 2018, 19(12): 3882. DOI:10.3390/ijms19123882 (  0) 0) |
| [33] |
WANG H, PAN L, ZHANG X, et al. The molecular mechanism of AhR-ARNT-XREs signaling pathway in the detoxification response induced by polycyclic aromatic hydrocarbons (PAHs) in clam Ruditapes philippinarum[J]. Environmental Research, 2020, 183: 109165. DOI:10.1016/j.envres.2020.109165 (  0) 0) |
| [34] |
ESSER C, RANNUG A. The aryl hydrocarbon receptor in barrier organ physiology, immunology, and toxicology[J]. Pharmacological Reviews, 2015, 67(2): 259-279. DOI:10.1124/pr.114.009001 (  0) 0) |
| [35] |
BAKER J R, SAKOFF J A, MCCLUSKEY A. The aryl hydrocarbon receptor (AhR) as a breast cancer drug target[J]. Medicinal Research Reviews, 2020, 40(3): 972-1001. DOI:10.1002/med.21645 (  0) 0) |
| [36] |
MICHALICKOVA D, HRNCIR T, CANOVA N K, et al. Targeting Keap1/Nrf2/ARE signaling pathway in multiple sclerosis[J]. European Journal of Pharmacology, 2020, 873: 172973. DOI:10.1016/j.ejphar.2020.172973 (  0) 0) |
| [37] |
GOMEZ M A, CAJARAVILLE M P. Comparative effects of cadmium, copper, paraquat and benzo[a]pyrene on the actin cytoskeleton and production of reactive oxygen species (ROS) in mussel haemocytes[J]. Toxicology In Vitro, 2003, 17(5/6): 539-546. DOI:10.1016/s0887-2333(03)00093-6 (  0) 0) |
| [38] |
WANG Jun, ZHANG Wentong, LV Chao, et al. A novel biscoumarin compound ameliorates cerebral ischemia reperfusion-induced mitochondrial oxidative injury via Nrf2/Keap1/ARE signaling[J]. Neuropharmacology, 2020, 167: 107918. DOI:10.1016/j.neuropharm.2019.107918 (  0) 0) |
| [39] |
COPPLE I M, GOLDRING C E, NEIL K R, et al. The Nrf2-Keap1 defence pathway: role in protection against drug-induced toxicity[J]. Toxicology, 2008, 246(1): 24-33. DOI:10.1016/j.tox.2007.10.029 (  0) 0) |
| [40] |
YASUTAKE K, KEN I, EISAKU Y, et al. Two domains of Nrf2 cooperatively bind CBP, a CREB binding protein, and synergistically activate transcription[J]. Genes Cells, 2001, 6(10): 857-868. DOI:10.1046/j.1365-2443.2001.00469.x (  0) 0) |
| [41] |
TAGUCHI K, MOTOHASHI H, YAMAMOTO M. Molecular mechanisms of the Keap1-Nrf2 pathway in stress response and cancer evolution[J]. Genes Cells, 2011, 16(2): 123-140. DOI:10.1111/j.1365-2443.2010.01473.x (  0) 0) |
| [42] |
CHEN Q M, MALTAGLIATI A J. Nrf2 at the heart of oxidative stress and cardiac protection[J]. Physiological Genomics, 2018, 50(2): 77-97. DOI:10.1152/physiolgenomics.00041.2017 (  0) 0) |
| [43] |
BANNING A, BRIGELIUS FLOHE R. NF-kappaB, Nrf2, and HO-1 interplay in redox-regulated VCAM-1 expression[J]. Antioxidants & Redox Signaling, 2005, 7(7/8): 889-899. DOI:10.1089/ars.2005.7.889 (  0) 0) |
| [44] |
STORM J, XU B, TIAN X, et al. Nrf2 protects mitochondrial decay by oxidative stress[J]. FASEB Journal, 2016, 30(1): 66-80. DOI:10.1096/fj.14-268904 (  0) 0) |
| [45] |
TELORACK M, MEYER M, INGOLD I, et al. A glutathione-Nrf2-thioredoxin cross-talk ensures keratinocyte survival and efficient wound repair[J]. PLoS Genetics, 2016, 12(1): e1005800. DOI:10.1371/journal.pgen.1005800 (  0) 0) |
| [46] |
HARRIS I S, DENICOLA G M. The complex interplay between antioxidants and ROS in cancer[J]. Trends in Cell Biology, 2020, 30(6): 440-451. DOI:10.1016/j.tcb.2020.03.002 (  0) 0) |
| [47] |
DENICOLA G M, KARRETH F A, HUMPTON T J, et al. Oncogene-induced Nrf2 transcription promotes ROS detoxification and tumorigenesis[J]. Nature, 2011, 475(7354): 106-109. DOI:10.1038/nature10189 (  0) 0) |
| [48] |
SHAH S Z A, ZHAO D, HUSSAIN T, et al. p62-Keap1-NRF2-ARE pathway: A contentious player for selective targeting of autophagy, oxidative stress and mitochondrial dysfunction in prion diseases[J]. Frontiers in Molecular Neuroscience, 2018, 11: 310. DOI:10.3389/fnmol.2018.00310 (  0) 0) |
| [49] |
SABITHA R, NISHI K, GUNASEKARAN V P, et al. p-Coumaric acid attenuates alcohol exposed hepatic injury through MAPKs, apoptosis and Nrf2 signaling in experimental models[J]. Chemico-Biological. Interactions, 2020, 321: 109044. DOI:10.1016/j.cbi.2020.109044 (  0) 0) |
| [50] |
LI J, LEE J M, JOHNSON J A. Microarray analysis reveals an antioxidant responsive element-driven gene set involved in conferring protection from an oxidative stress-induced apoptosis in IMR-32 cells[J]. The Journal of Biological Chemistry, 2002, 277(1): 388-394. DOI:10.1074/jbc.M109380200 (  0) 0) |
| [51] |
SINGH R, CHANDRASHEKHARAPPA S, BODDULURI S R, et al. Enhancement of the gut barrier integrity by a microbial metabolite through the Nrf2 pathway[J]. Nature Communications, 2019, 10(1): 89. DOI:10.1038/s41467-018-07859-7 (  0) 0) |
| [52] |
BOSE P, SIDDIQUE M, ACHARYA R, et al. Quinazolinone derivative BNUA-3 ameliorated[NDEA+2-AAF]-induced liver carcinogenesis in SD rats by modulating AhR-CYP1B1-Nrf2-Keap1 pathway[J]. Clinical and Experimental Pharmacology & Physiology, 2020, 47(1): 143-157. DOI:10.1111/1440-1681.13184 (  0) 0) |
| [53] |
LAWAL A O. Air particulate matter induced oxidative stress and inflammation in cardiovascular disease and atherosclerosis: The role of Nrf2 and AhR-mediated pathways[J]. Toxicology Letters, 2017, 270: 88-95. DOI:10.1016/j.toxlet.2017.01.017 (  0) 0) |
| [54] |
FURUE M, TAKAHARA M, NKAHARA T, et al. Role of AhR/ARNT system in skin homeostasis[J]. Archives of Dermatological Research, 2014, 306(9): 769-779. DOI:10.1007/s00403-014-1481-7 (  0) 0) |
| [55] |
ESSER C, BARGEN I, WEIGHARDT H, et al. Functions of the aryl hydrocarbon receptor in the skin[J]. Seminars in Immunopathology, 2013, 35(6): 677-691. DOI:10.1007/s00281-013-0394-4 (  0) 0) |
| [56] |
FUYUNO Y, UCHI H, YASUMATSU M, et al. Perillaldehyde inhibits AHR signaling and activates NRF2 antioxidant pathway in human keratinocytes[J]. Oxidative Medicine and Cellular Longevity, 2018, 14: 9524657. DOI:10.1155/2018/9524657 (  0) 0) |
| [57] |
SILVA P A, OSTOLGA C M, SANCHEZ G C, et al. Sulforaphane protects from myocardial ischemia-reperfusion damage through the balanced activation of Nrf2/AhR[J]. Free Radical Biology & Medicine, 2019, 143: 331-340. DOI:10.1016/j.freeradbiomed.2019.08.012 (  0) 0) |
| [58] |
RIE Y, EIKO K, TIN T W S, et al. Low-dose benzo[α]pyrene aggravates allergic airway inflammation in mice[J]. Journal of Applied Toxicology, 2016, 36(11): 1496-1504. DOI:10.1002/jat.3308 (  0) 0) |
| [59] |
YANG X H, LIU D H, MURRAY T J, et al. The aryl hydrocarbon receptor constitutively represses c-myc transcription in human mammary tumor cells[J]. Oncogene, 2005, 24(53): 7869-7881. DOI:10.1038/sj.onc.1208938 (  0) 0) |
| [60] |
SHIMBA S, WADA T, TEZUKA M. Arylhydrocarbon receptor (AhR) is involved in negative regulation of adipose differentiation in 3T3-L1 cells: AhR inhibits adipose differentiation independently of dioxin[J]. Journal of Cell Science, 2001, 114(15): 2809-2817. (  0) 0) |
| [61] |
ALEXANDER D L, GANEM L G, FERNANDEZ S P, et al. Aryl-hydrocarbon receptor is an inhibitory regulator of lipid synthesis and of commitment to adipogenesis[J]. Journal of Cell Science, 1998, 111(22): 3311-3322. (  0) 0) |
| [62] |
MA Q, KINNEER K, BI Y, et al. Induction of murine NAD(P)H: quinone oxidoreductase by 2, 3, 7, 8-tetrachlorodibenzo-p-dioxin requires the CNC (cap 'n' collar) basic leucine zipper transcription factor Nrf2 (nuclear factor erythroid 2-related factor 2): cross-interaction between AhR (aryl hydrocarbon receptor) and Nrf2 signal transduction[J]. The Biochemical Journal, 2004, 377(1): 205-213. DOI:10.1042/BJ20031123 (  0) 0) |
| [63] |
SHIN S, WAKABAYASHI N, MISRA V, et al. NRF2 modulates aryl hydrocarbon receptor signaling: influence on adipogenesis[J]. Molecular and Cellular Biology, 2007, 27(20): 7188-7197. DOI:10.1128/MCB.00915-07 (  0) 0) |
| [64] |
MIAO W M, HU L G, SCRIVENS P J, et al. Transcriptional regulation of NF-E2 p45-related factor (NRF2) expression by the aryl hydrocarbon receptor-xenobiotic response element signaling pathway: direct cross-talk between phase Ⅰ and Ⅱ drug-metabolizing enzymes[J]. The Journal of Biological Chemistry, 2005, 280(21): 20340-20348. DOI:10.1074/jbc.M412081200 (  0) 0) |
| [65] |
KOHLE C, BOCK K W. Coordinate regulation of Phase Ⅰ and Ⅱ xenobiotic metabolisms by the Ah receptor and Nrf2[J]. Biochemical Pharmacology, 2007, 73(12): 1853-1862. DOI:10.1016/j.bcp.2007.01.009 (  0) 0) |
| [66] |
LIN X H, YANG H, ZHOU L C, et al. Nrf2-dependent induction of NQO1 in mouse aortic endothelial cells overexpressing catalase[J]. Free Radical Biology & Medicine, 2011, 51(1): 97-106. DOI:10.1016/j.freeradbiomed.2011.04.020 (  0) 0) |
| [67] |
NAKAHARA T, MITOMA C, HASHIMOTO H A, et al. Antioxidant opuntia ficus-indica extract activates AHR-NRF2 signaling and upregulates filaggrin and loricrin expression in human keratinocytes[J]. Journal of Medicinal Food, 2015, 18(10): 1143-1149. DOI:10.1089/jmf.2014.3396 (  0) 0) |
| [68] |
LAWAL A O. Air particulate matter induced oxidative stress and inflammation in cardiovascular disease and atherosclerosis: The role of Nrf2 and AhR-mediated pathway[J]. Toxicology Letters, 2017, 270(15): 88-95. DOI:10.1016/j.toxlet.2017.01.017 (  0) 0) |
| [69] |
YUAN L L, DUAN X W, ZHANG R T, et al. Aloe polysaccharide protects skin cells from UVB irradiation through Keap1/Nrf2/ARE signal pathway[J]. The Journal of Dermatological Treatment, 2020, 31(3): 300-308. DOI:10.1080/09546634.2019.1591579 (  0) 0) |
| [70] |
CHRISTOPH K, KARL W B. Coordinate regulation of Phase Ⅰ and Ⅱ xenobiotic metabolisms by the Ah receptor and Nrf2[J]. Biochemical Pharmacology, 2007, 73(12): 1853-1862. DOI:10.1016/j.bcp.2007.01.009 (  0) 0) |
| [71] |
ALBERT P S, TIMOTHY P D, DANIEL W N, et al. Dioxin increases reactive oxygen production in mouse liver mitochondria[J]. Toxicology and Applied Pharmacology, 2002, 178(1): 15-21. DOI:10.1006/taap.2001.9314 (  0) 0) |
| [72] |
TOYDEMIR G, LOONEN L M P, VENKATASUBRAMANIAN P B, et al. Coffee induces AHR-and Nrf2-mediated transcription in intestinal epithelial cells[J]. Food Chemistry, 2020, 341(2): 128261. DOI:10.1016/j.foodchem.2020.128261 (  0) 0) |
| [73] |
HIEMSTRA S, NIEMEIJER M, KOEDOOT E, et al. Comprehensive landscape of Nrf2 and p53 pathway activation dynamics by oxidative stress and DNA damage[J]. Chemical Research in Toxicology, 2017, 30(4): 923-933. DOI:10.1021/acs.chemrestox.6b00322 (  0) 0) |
| [74] |
LEE S, LIM M J, KIM M H, et al. An effective strategy for increasing the radiosensitivity of Human lung Cancer cells by blocking Nrf2-dependent antioxidant responses[J]. Free Radical Biology & Medicine, 2012, 53(4): 807-816. DOI:10.1016/j.freeradbiomed.2012.05.038 (  0) 0) |
| [75] |
SHI L, HAO Z, ZHANG S, et al. Baicalein and baicalin alleviate acetaminophen-induced liver injury by activating Nrf2 antioxidative pathway: The involvement of ERK1/2 and PKC[J]. Biochemical Pharmacology, 2018, 150: 9-23. DOI:10.1016/j.bcp.2018.01.026 (  0) 0) |
| [76] |
HAYES J D, MCLELLAN L I. Glutathione and glutathione-dependent enzymes represent a co-ordinately regulated defence against oxidative stress[J]. Free Radical Research, 1999, 31(4): 273-300. DOI:10.1080/10715769900300851 (  0) 0) |
| [77] |
LEUY S, JAISWAL A K, FORMAN H J. The role of c-Jun phosphorylation in EpRE activation of phase Ⅱ genes[J]. Free Radical Biology & Medicine, 2009, 47(8): 1172-1179. DOI:10.1016/j.freeradbiomed.2009.07.036 (  0) 0) |
| [78] |
KAY H Y, KIM Y W, RYU D H, et al. Nrf2-mediated liver protection by sauchinone, an antioxidant lignan, from acetaminophen toxicity through the PKCδ-GSK3β pathway[J]. British Journal of Pharmacology, 2011, 163(8): 1653-1665. DOI:10.1111/j.1476-5381.2010.01095.x (  0) 0) |
| [79] |
MENEGON S, COLUMBANO A, GIORDANO S. The dual roles of NRF2 in cancer[J]. Trends in Molecular Medicine, 2016, 22(7): 578-593. DOI:10.1016/j.molmed.2016.05.002 (  0) 0) |
| [80] |
GEGOTEK A, SKRZYDLEWSKA E. The role of transcription factor Nrf2 in skin cells metabolism[J]. Archives of Dermatological Research, 2015, 307(5): 385-396. DOI:10.1007/s00403-015-1554-2 (  0) 0) |
| [81] |
RAMAKRISHNAN S, KUMARI N, VINOTH P G, et al. p-Coumaric acid attenuates alcohol exposed hepatic injury through MAPKs, apoptosis and Nrf2 signaling in experimental models[J]. Chemico-Biological Interactions, 2020, 321: 109044. DOI:10.1016/j.cbi.2020.109044 (  0) 0) |
 2022, Vol. 20
2022, Vol. 20


