2. 湖南大学 深圳研究院,广东 深圳 518057
2. Shenzhen Institute, Hunan University, Shenzhen 518057, Guangdong, China
植物的生长受到多种环境因素的影响,如温度、湿度、光照等。光不仅是能量来源,也作为周围环境的一部分影响植物的生长发育。植物能够通过各类光受体敏锐地感知光质和光强的变化,进而适应环境的变化。植物体内有一类红光/远红光受体:光敏色素[1](Phytochromes)和两类蓝光受体:隐花色素[2]和向光素[3]。在拟南芥中,主要含有两种同源的隐花色素,CRY1和CRY2。CRY1主要参与蓝光对下胚轴伸长的抑制作用[4],CRY2则主要参与调控植物的开花时间[5]。拟南芥的向光素也有两种,即phot1和phot2,主要参与控制植物的向光性[3]。F-box蛋白家族成员ZEITLUPE (ZTL),FLAVIN-BINDING KELCH REPEAT F-BOX1 (FKF1)和LOV KELCH PROTEIN2 (LKP2)含有与向光素高度类似的LOV(Light, Oxygen, or Voltage)结构域[6],能够吸收蓝光,被证明作为蓝光受体在植物体内发挥作用[7-9]。本文对ZTL/FKF1/LKP2蛋白家族的生物学功能研究进展进行综述,探讨其作用机制。
1 ZTL/FKF1/LKP2蛋白家族的结构ZTL/FKF1/LKP2蛋白家族包含了三个功能结构域:LOV (Light, Oxygen, or Voltage)、F-box和Kelch repeats(见图 1)[10]。LOV结构域是一个小的光感原件,属于PAS结构域大家族中的一员[11]。ZTL/FKF1/LKP2家族成员的LOV结构域通过结合黄素单核苷酸(其作为发色团)而参与蓝光感知。F-box结构域通过与ASK蛋白家族成员结合参与形SKP1/CUL1/F-box (SCF)泛素连接酶复合体[12]。kelch重复结构域由kelch基序的5~7个串联重复组成,并形成β-螺旋桨结构, 是典型的蛋白互作结构域[13]。F-box蛋白在SCF复合体中通过其kelch结构域参与识别和结合靶蛋白,促进靶蛋白被泛素化降解[14]。ZTL/FKF1/LKP2蛋白家族在植物体内通常通过其F-box结构域和kelch结构域,行使SCF泛素连接酶的功能[15-16]。因此,这三个功能结构域赋予ZTL/FKF1/LKP2即能作为蓝光受体在植物体内参与光信号传导,也具有F-box蛋白的功能。ZTL/FKF1/LKP2通常介导靶蛋白以蓝光依赖的方式通过泛素蛋白酶体途径降解,进而调控植物的生长发育。
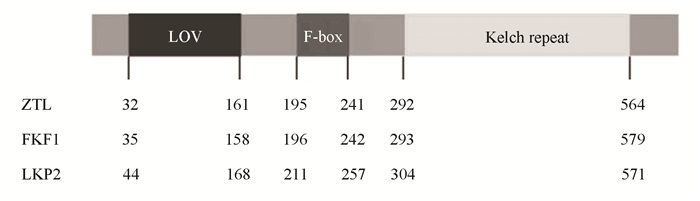
|
图 1 ZTL/FKF1/LKP2的蛋白结构 Figure 1 Protein structures of ZTL/FKF1/LKP2 |
ZTL/FKF1/LKP2蛋白家族虽然在氨基酸序列上具有很高的相似性(70%~80%)[7-9],但三者并没有表现出很明显的功能重叠,在植物体内的功能差异较大。例如:ZTL在生物钟的调控中更为关键;FKF1则在光周期开花调控中的作用更为显著[7-8];LKP2在生物钟调控中也扮演着重要的角色[17]。因为这些相似与差异,三者的作用机制也更为复杂。
2.1 ZTL/FKF1/LKP2蛋白家族参与调控生物钟植物生物钟振荡受到多种转录因子和蛋白的调控,包括TIMING OF CAB EXPRESSION1(TOC1,也叫做PRR1)、CIRCADIAN CLOCK ASSOCIATED1 (CCA1)和LATE ELONGATED HYPOCOTYL (LHY)。这些转录因子和蛋白构成多个负反馈循环,组成中心振荡器,共同维持生物钟的正常运行[18-19]。例如:TOC1在夜晚表达,能够在黎明通过直接调节转录因子CCA1 HIKING EXPEDITION(CHE)的活性,使CCA1和LHY的表达增加,而CCA1和LHY则负调节TOC1的表达,从而形成负反馈循环共同调控生物钟振荡[20-22]。在拟南芥中,ZTL/FKF1/LKP2蛋白家族主要通过调节生物钟蛋白的稳定性,参与调控生物钟振荡。ZTL的LOV结构域能够特异的结合TOC1和PSEUDO RESPONSE REGULATOR 5 (PRR5),进而促进这些蛋白通过泛素蛋白酶体途径降解(见图 2)[23]。TOC1和PRR5蛋白在ztl突变体中积累,在ZTL过表达株系中则减少[24]。在白天,蓝光诱导ZTL与GIGANTEA(GI)结合,从而保护TOC1和PRR5免于被ZTL降解(见图 2)[25]。虽然LKP2和FKF1都能够与TOC1和PRR5结合,但是其作用较ZTL弱[23]。最近研究发现,ZTL与CHE直接相互作用,介导CHE的泛素化降解(见图 2)。在黑暗中,CHE蛋白降解,而在ztl突变植物中降解速度变慢[14]。此外,ZTL也可通过感知蓝光强度的变化来调控植物的生物钟振荡[26]。
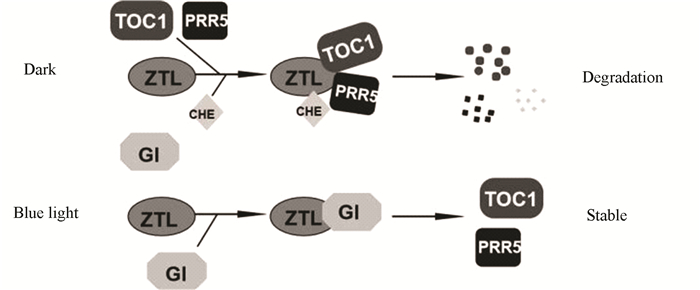
|
图 2 ZTL/FKF1/LKP2光介导调控生物钟蛋白的稳定性 Figure 2 ZTL/FKF1/LKP2 mediate light regulation of circadian clock proteins stability |
Somers等[8]2000年报道,长日照(LD)条件下,ztl突变体表现出明显的长周期表型,而fkf1突变体则没有明显的生物钟周期缺陷[23]。lkp2功能缺失突变体的生物节律与野生型也基本一致[27]。与ztl单突变体相比,lkp2 ztl双突变体的周期节律并没有明显变化,而fkf1 ztl则表现出较长的周期[28]。在红光或蓝光下,将ZTL的LOV或LOV-F-box结构域过表达导致昼夜节律延长[25]。LKP2的LOV或LOV-F-box结构域过表达植株则表现出更短的周期[17]。ZTL LOV和LKP2 LOV对生物钟调控的不同作用可能是由于它们的相互作用蛋白不同,或者对相互作用蛋白的亲和力不同有关,还有待进一步研究。
2.2 ZTL/FKF1/LKP2蛋白家族在光周期开花途径中的功能植物开花时间受到多种内外源因素精确调控,形成了光周期、自主开花、赤霉素和春化多种开花途径[29]。拟南芥是长日照植物,长日照诱导其开花,短日照则抑制其开花。锌指型转录因子CONSTANS(CO)对FLOWERING LOCUS T (FT)基因表达量的调控,在拟南芥光周期开花途径中至关重要[30]。长日照下,CO直接结合FT的启动子,促进FT的表达,进而促进开花[31]。ZTL、FKF1和LKP2通过直接调控CO蛋白的稳定性,或者调控CO基因的转录水平,进而调控光周期开花。ZTL在长日照中介导CO蛋白在早上降解[32];FKF1则在长日照中通过其LOV结构域与CO蛋白结合,维持CO蛋白在傍晚的稳定性,进而促进FT的表达(见图 3)[33]。FKF1/ZTL/LKP2还协同介导转录因子CYCLING DOF FACTOR 1 (CDF1)和CDF2蛋白降解,解除CDF1和CDF2对CO转录的抑制作用,诱导CO转录表达,从而促进开花(见图 3)[34-36]。此外,光依赖的FKF1-CONSTITUTIVE PHOTOMORPHOGENIC1 (COP1)相互作用,通过抑制E3泛素连接酶COP1的活性,解除COP1对CO蛋白的降解,进而促进FT的表达和开花(见图 3)[37-38]。最近研究发现,FKF1与赤霉素信号途径关键负调节子DELLA蛋白互作,部分通过促进DELLA蛋白降解进而促进植物开花(结果未发表)。此外,ztl突变还导致花开放、香味散发、花梗运动的节律发生改变[39]。
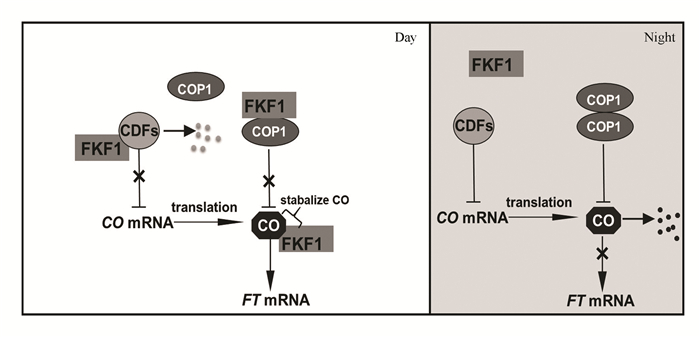
|
图 3 FKF1在光周期开花途径中的功能 Figure 3 Function of FKF1 in the control of photoperiodic flowering |
在光周期开花信号通路中,虽然ZTL/FKF1/LKP2有多个相同的靶蛋白,但其中起主导作用的是FKF1。长日照下,fkf1突变体表现出明显的晚花表型[36]。短日照下,ztl突变体表现微弱的早花表型;在短日照和长日照下,lkp2突变体无明显开花表型,lkp2 ztl双突体则表现出比ztl单突变体更明显的早花表型,且这种早花表型依赖于FKF1[24]。相反,过表达ZTL或者LKP2下调了CO和FT的表达,导致植物在长日照下开花延迟,这种晚花表型也依赖于FKF1[24]。ZTL和LKP2主要通过调控FKF1蛋白定位和蛋白水平,进而调控植物光周期开花[24, 40]。
2.3 ZTL/FKF1/LKP2蛋白家族的其它功能ZTL/FKF1/LKP2除了影响植物光周期开花以及生物钟节律外,在植物光形态建成、逆境胁迫响应中也起重要调控作用。LKP2促进白光下拟南芥幼苗下胚轴伸长。LKP2过表达导致拟南芥幼苗中生长素IAA水平升高,PHYTOCHROME-INTERACTING FACTOR4 (PIF4)和PIF5的表达水平上调,进而使下胚轴伸长[41]。ZTL也促进白光下拟南芥幼苗下胚轴伸长,并且在高温诱导的下胚轴伸长中起正调控作用[42]。fkf1突变体在红光和蓝光下则表现出短下胚轴的表型,因此FKF1在光形态建成中可能起负调控作用[7]。Gil等[43]2017年研究发现ZTL能够与Heat shock protein 90(HSP90)互作,介导响应高温而形成的蛋白聚集体的泛素化降解,控制蛋白的质量,从而稳定生物钟并增强植物的耐热性。此外,LKP2在植物干旱胁迫响应中起正调控作用,LKP2过表达使植物气孔孔径变小,干旱胁迫响应基因表达量上升,耐旱性增强[44]。
3 展望近年来,关于ZTL/FKF1/LKP2的研究更多的关注于其在植物生物钟和光周期开花途径中的功能及其调控机制。ZTL/FKF1/LKP2也参与调控植物其它生物学过程,包括光形态建成和逆境胁迫响应,但其作用机制尚不完全明确。已有的基因芯片结果显示,LKP2过表达引起了众多信号通路基因的改变,尤其是生长素信号通路相关基因表达发生明显改变[44]。目前,有研究报道,蓝光受体CRY1和红光/远红光受体PHYB /PHYA能够与AUX/IAA蛋白互作来影响生长素的信号转导,调控植物的生长发育[45-46]。同时,CRY1能够通过调节油菜素内酯(BR)的合成[47]与信号转导[48-49]来影响植物的光形态建成。最新研究表明,紫外光UV-B与BR信号协同调控下胚轴伸长[50]。因此,ZTL/FKF1/LKP2也可能在光信号通路与激素信号通路的交叉会话中发挥作用,这为我们今后的研究提供新的思路。
| [1] |
FRANKLIN K A, QUAIL P H. Phytochrome functions in Arabidopsis development[J]. Journal of Experimental Botany, 2010, 61(1): 11-24. DOI:10.1093/jxb/erp304 (  0) 0) |
| [2] |
CHAVES I, POKORNY R, BYRDIN M, et al. The cryptochromes: Blue light photoreceptors in plants and animals[J]. Annual Review of Plant Biology, 2011, 62(1): 335-364. DOI:10.1146/annurev-arplant-042110-103759 (  0) 0) |
| [3] |
CHRISTIE J, JONES M, SULLIVAN S, et al. Structure-function analysis of phototropin blue-light receptors[J]. Comparative Biochemistry and Physiology Part A Molecular & Integrative Physiology, 2007, 146(4): 21-45. DOI:10.1016/j.cbpa.2007.01.499 (  0) 0) |
| [4] |
AHMAD M, CASHMORE A R. HY4 gene of A.thaliana encodes a protein with characteristics of a blue-light photoreceptor[J]. Nature, 1993, 366(6451): 162-166. DOI:10.1038/366162a0 (  0) 0) |
| [5] |
GUO H, YANG H, MOCKLER T C, et al. Regulation of flowering time by Arabidopsis photoreceptors[J]. Science, 1998, 279(5355): 1360-1363. DOI:10.1126/science.279.5355.1360 (  0) 0) |
| [6] |
CHRISTIE J M, REYMOND P, POWELL G K, et al. Arabidopsis NPH1: A flavoprotein with the properties of a photoreceptor for phototropism[J]. Science, 1998, 282(5394): 1698-1701. DOI:10.1126/science.282.5394.1698 (  0) 0) |
| [7] |
NELSON D C, LASSWELL J, ROGG L E, et al. FKF1, a clock-controlled gene that regulates the transition to flowering in Arabidopsis[J]. Cell, 2000, 101(3): 331-340. DOI:10.1016/S0092-8674(00)80842-9 (  0) 0) |
| [8] |
SOMERS D E, SCHULTZ T F, MILNAMOW M, et al. ZEITLUPE encodes a novel clock-associated PAS protein from Arabidopsis[J]. Cell, 2000, 101(3): 319-329. DOI:10.1016/S0092-8674(00)80841-7 (  0) 0) |
| [9] |
SCHULTZ T F, KIYOSUE T, YANOVSKY M, et al. A role for LKP2 in the circadian clock of Arabidopsis[J]. The Plant Cell, 2001, 13(12): 2659-2670. DOI:10.1105/tpc.010332 (  0) 0) |
| [10] |
ZOLTOWSKI B D, IMAIZUMI T. Chapter nine-structure and function of the ZTL/FKF1/LKP2 group proteins in Arabidopsis[J]. Enzymes, 2014, 35: 213-239. DOI:10.1016/B978-0-12-801922-1.00009-9 (  0) 0) |
| [11] |
TAYLOR B L, ZHULIN I B. PAS domains: Internal sensors of oxygen, redox potential, and light[J]. Microbiology and Molecular Biology Reviews, 1999, 63(2): 479-506. (  0) 0) |
| [12] |
KIPREOS E T, PAGANO M. The F-box protein family[J]. Genome Biology, 2000, 1(5): 1-7. DOI:10.1186/gb-2000-1-5-reviews3002 (  0) 0) |
| [13] |
ADAMS J, KELSO R, COOLEY L. The kelch repeat superfamily of proteins: Propellers of cell function[J]. Trends in Cell Biology, 2000, 10(1): 17-24. DOI:10.1016/S0962-8924(99)01673-6 (  0) 0) |
| [14] |
ANDRADE M A, GONZÁLEZ-GUZMÁN M, Serrano R, et al. A combination of the F-box motif and kelch repeats defines a large Arabidopsis family of F-box proteins[J]. Plant Molecular Biology, 2001, 46(5): 603-614. DOI:10.1023/A:1010650809272 (  0) 0) |
| [15] |
HAN L, MASON M, RISSEEUW E P, et al. Formation of an SCFZTL complex is required for proper regulation of circadian timing[J]. The Plant Journal, 2004, 40(2): 291-301. DOI:10.1111/j.1365-313X.2004.02207.x (  0) 0) |
| [16] |
LEE C M, FEKE A, LI M W, et al. Decoys untangle complicated redundancy and reveal targets of circadian clock F-box proteins[J]. Plant Physiology, 2018, 177(3): 1170-1186. DOI:10.1104/pp.18.00331 (  0) 0) |
| [17] |
TAKASE T, MIYAZAKI Y, YASUHARA M, et al. Pleiotropic phenotype of transgenic Arabidopsis plants that produce the LOV domain of LOV KELCH PROTEIN2 (LKP2)[J]. Plant Biotechnology, 2015, 32(4): 273-280. DOI:10.5511/plantbiotechnology.15.0808b (  0) 0) |
| [18] |
MÁS P. Circadian clock function in Arabidopsis thaliana: Time beyond transcription[J]. Trends in Cell Biology, 2008, 18(6): 273-281. DOI:10.1016/j.tcb.2008.03.005 (  0) 0) |
| [19] |
HARMER S L. The circadian system in higher plants[J]. Annual Review of Plant Biology, 2009, 60(1): 357-377. DOI:10.1146/annurev.arplant.043008.092054 (  0) 0) |
| [20] |
MAKINO S, MATSUSHIKA A, KOJIMA M, et al. The APRR1/TOC1 quintet implicated in circadian rhythms of Arabidopsis thaliana: I. characterization with APRR1-overexpressing plants[J]. Plant and Cell Physiology, 2002, 3(1): 58-69. DOI:10.1093/pcp/pcf005 (  0) 0) |
| [21] |
PRUNEDA-PAZ J L, BRETON G, PARA A, et al. A functional genomics approach reveals CHE as a component of the Arabidopsis circadian clock[J]. Science, 2009, 323(5920): 1481-1485. DOI:10.1126/science.1167206 (  0) 0) |
| [22] |
ALABADÍ D, OYAMA T, YANOVSKY M J, et al. Reciprocal regulation between TOC1 and LHY/CCA1 within the Arabidopsis circadian clock[J]. Science, 2001, 293(5531): 880-883. DOI:10.1126/science.1061320 (  0) 0) |
| [23] |
BAUDRY A, ITO S, SONG Y H, et al. F-box proteins FKF1 and LKP2 act in concert with ZEITLUPE to control Arabidopsis clock progression[J]. The Plant Cell, 2010, 22(3): 606-622. DOI:10.1105/tpc.109.072843 (  0) 0) |
| [24] |
TAKASE T, NISHIYAMA Y, TANIHIGASHI H, et al. LOV KELCH PROTEIN2 and ZEITLUPE repress Arabidopsis photoperiodic flowering under non-inductive conditions, dependent on FLAVIN-BINDING KELCH REPEAT F-BOX1[J]. The Plant Journal, 2011, 67(4): 608-621. DOI:10.1111/j.1365-313X.2011.04618.x (  0) 0) |
| [25] |
KIM W Y, FUJIWARA S, SUH S S, et al. ZEITLUPE is a circadian photoreceptor stabilized by GIGANTEA in blue light[J]. Nature, 2007, 449(7160): 356-360. DOI:10.1038/nature06132 (  0) 0) |
| [26] |
PUDASAINI A, ZOLTOWSKI B D. Zeitlupe senses blue-light fluence to mediate circadian timing in Arabidopsis thaliana[J]. Biochemistry, 2013, 52(40): 7150-7158. DOI:10.1021/bi401027n (  0) 0) |
| [27] |
ANDRÉ S F, COUPLAND G. The genetic basis of flowering responses to seasonal cues[J]. Nature Reviews Genetics, 2012, 13(9): 627. DOI:10.1038/nrg3291 (  0) 0) |
| [28] |
SUÁREZ-LÓPEZ P, WHEATLEY K, ROBSON F, et al. CONSTANS mediates between the circadian clock and the control of flowering in Arabidopsis[J]. Nature, 2001, 410: 1116. DOI:10.1038/35074138 (  0) 0) |
| [29] |
TIWARI S B, SHEN Y, CHANG H C, et al. The flowering time regulator CONSTANS is recruited to the FLOWERING LOCUS T promoter via a unique cis-element[J]. New Phytologist, 2010, 187(1): 57-66. DOI:10.1111/j.1469-8137.2010.03251.x (  0) 0) |
| [30] |
HOLM M, HARDTKE C S, GAUDET R, et al. Identification of a structural motif that confers specific interaction with the WD40 repeat domain of Arabidopsis COP1[J]. The EMBO Journal, 2001, 20(1-2): 118-127. DOI:10.1093/emboj/20.1.118 (  0) 0) |
| [31] |
LIU Lijun, ZHANG Yanchun, LI Qinghua, et al. COP1-Mediated ubiquitination of CONSTANS is implicated in cryptochrome regulation of flowering in Arabidopsis[J]. The Plant Cell, 2008, 20(2): 292-306. DOI:10.1105/tpc.107.057281 (  0) 0) |
| [32] |
SONG Y H, ESTRADA D A, JOHNSON R S, et al. Distinct roles of FKF1, GIGANTEA, and ZEITLUPE proteins in the regulation of CONSTANS stability in Arabidopsis photoperiodic flowering[J]. Proceedings of the National Academy of Sciences, 2014, 111(49): 17672-17677. DOI:10.1073/pnas.1415375111 (  0) 0) |
| [33] |
SONG Y H, LEE I, LEE S Y, et al. CONSTANS and ASYMMETRIC LEAVES 1 complex is involved in the induction of FLOWERING LOCUS T in photoperiodic flowering in Arabidopsis[J]. The Plant Journal, 2012, 69(2): 332-342. DOI:10.1111/j.1365-313X.2011.04793.x (  0) 0) |
| [34] |
IMAIZUMI T, SCHULTZ T F, HARMON F G, et al. FKF1 F-box protein mediates cyclic degradation of a repressor of CONSTANS in Arabidopsis[J]. Science, 2005, 309(5732): 293-297. DOI:10.1126/science.1110586 (  0) 0) |
| [35] |
SAWA M, NUSINOW D A, KAY S A, et al. FKF1 and GIGANTEA complex formation is required for day-length measurement in Arabidopsis[J]. Science, 2007, 318(5848): 261-265. DOI:10.1126/science.1146994 (  0) 0) |
| [36] |
FORNARA F, PANIGRAHI K C S, Gissot L, et al. Arabidopsis DOF transcription factors act redundantly to reduce CONSTANS expression and are essential for a photoperiodic flowering response[J]. Developmental Cell, 2009, 17(1): 75-86. DOI:10.1016/j.devcel.2009.06.015 (  0) 0) |
| [37] |
LEE B D, KIM M R, KANG M Y, et al. The F-box protein FKF1 inhibits dimerization of COP1 in the control of photoperiodic flowering[J]. Nature Communications, 2017, 8(1): 2259. DOI:10.1038/s41467-017-02476-2 (  0) 0) |
| [38] |
LEE B D, CHA J Y, KIM M R, et al. Light-dependent suppression of COP1 multimeric complex formation is determined by the blue-light receptor FKF1 in Arabidopsis[J]. Biochemical and Biophysical Research Communications, 2019, 508(1): 191-197. DOI:10.1016/j.bbrc.2018.11.032 (  0) 0) |
| [39] |
YON F, JOO Y, CORTÉS LLORCA L, et al. Silencing Nicotiana attenuata LHY and ZTL alters circadian rhythms in flowers[J]. New Phytologist, 2016, 209(3): 1058-1066. DOI:10.1111/nph.13681 (  0) 0) |
| [40] |
SOMERS D E, KIM W Y, GENG R. The F-Box protein ZEITLUPE confers dosage-Dependent control on the circadian clock, photomorphogenesis, and flowering time[J]. The Plant Cell, 2004, 16(3): 769-782. DOI:10.1105/tpc.016808 (  0) 0) |
| [41] |
MIYAZAKI Y, JIKUMARU Y, TAKASE T, et al. Enhancement of hypocotyl elongation by LOV KELCH PROTEIN2 production is mediated by auxin and phytochrome-interacting factors in Arabidopsis thaliana[J]. Plant Cell Reports, 2016, 35(2): 455-467. DOI:10.1007/s00299-015-1896-4 (  0) 0) |
| [42] |
MIYAZAKI Y, TAKASE T, KIYOSUE T. ZEITLUPE positively regulates hypocotyl elongation at warm temperature under light in Arabidopsis thaliana[J]. Plant Signaling & Behavior, 2015, 10(5): e998540. DOI:10.1080/15592324.2014.998540 (  0) 0) |
| [43] |
GIL K E, KIM W Y, LEE H J, et al. ZEITLUPE contributes to a thermoresponsive protein quality control system in Arabidopsis[J]. The Plant Cell, 2017, 29(11): 2882-2894. DOI:10.1105/tpc.17.00612 (  0) 0) |
| [44] |
MIYAZAKI Y, ABE H, TAKASE T, et al. Overexpression of LOV KELCH PROTEIN 2 confers dehydration tolerance and is associated with enhanced expression of dehydration-inducible genes in Arabidopsis thaliana[J]. Plant Cell Reports, 2015, 34(5): 843-852. DOI:10.1007/s00299-015-1746-4 (  0) 0) |
| [45] |
XU Feng, HE Shengbo, ZHANG Jingyi, et al. Photoactivated CRY1 and phyB interact directly with AUX/IAA proteins to inhibit auxin signaling in Arabidopsis[J]. Molecular Plant, 2017, 11(4): 523-541. DOI:10.1016/j.molp.2017.12.003 (  0) 0) |
| [46] |
YANG C, XIE F, JIANG Y, et al. Phytochrome a negatively regulates the shade avoidance response by increasing auxin/indole acidic acid protein stability[J]. Developmental Cell, 2018, 44(1): 29-41. DOI:10.1016/j.devcel.2017.11.017 (  0) 0) |
| [47] |
SAKAGUCHI J, MATSUSHITA T, WATANABE Y. DWARF4 accumulation in root tips is enhanced via blue light perception by cryptochromes[J]. Plant, Cell & Environment, 2019, 42(5): 1615-1629. DOI:10.1111/pce.13510 (  0) 0) |
| [48] |
WANG W, LU X, LI L, et al. Photoexcited CRYPTOCHROME1 interacts with dephosphory-lated BES1 to regulate brassinosteroid signaling and photomorphogenesis in Arabidopsis[J]. The Plant Cell, 2018, 30(9): 1989-2005. DOI:10.1105/tpc.17.00994 (  0) 0) |
| [49] |
HE Guanhua, LIU Jie, DONG Huixue, et al. The blue light receptor CRY1 interacts with BZR1 and BIN2 to modulate the phosphorylation and nuclear function of BZR1 in repressing BR signaling in Arabidopsis[J]. Molecular Plant, 2019, 12(5): 689-703. DOI:10.1016/j.molp.2019.02.001 (  0) 0) |
| [50] |
LIANG Tong, MEI Shenglin, SHI Chen, et al. UVR8 interacts with BES1 and BIM1 to regulate transcription and photomorphogenesis in Arabidopsis[J]. Develop Mental Cell, 2018, 44(4): 512-523.e5. DOI:10.1016/j.devcel.2017.12.028 (  0) 0) |
 2019, Vol. 17
2019, Vol. 17


